MCAT® General Chemistry
Topics, Practice Questions, and Study Strategies
Explore key MCAT® General Chemistry concepts, sharpen your skills with practice questions, and discover winning study strategies with UWorld. Prepare to ace your MCAT and achieve your medical career goals!

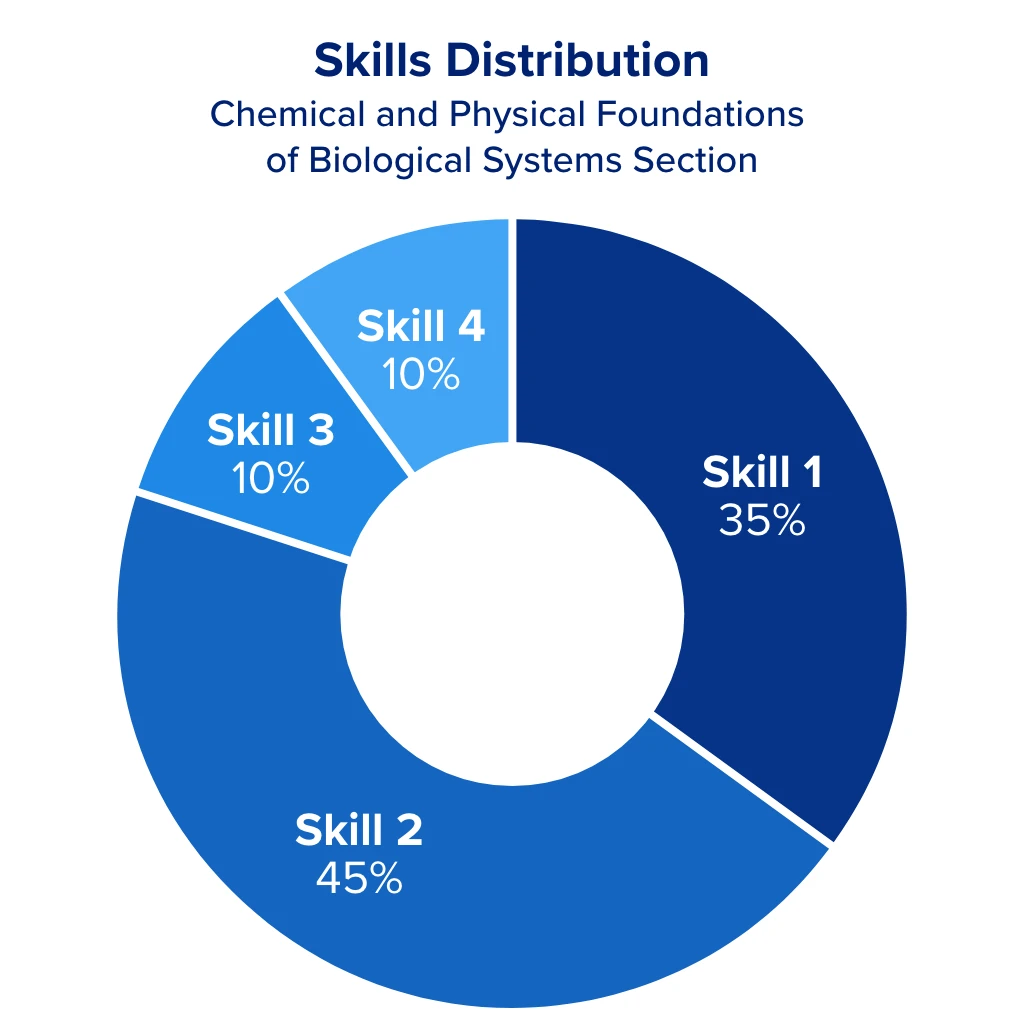
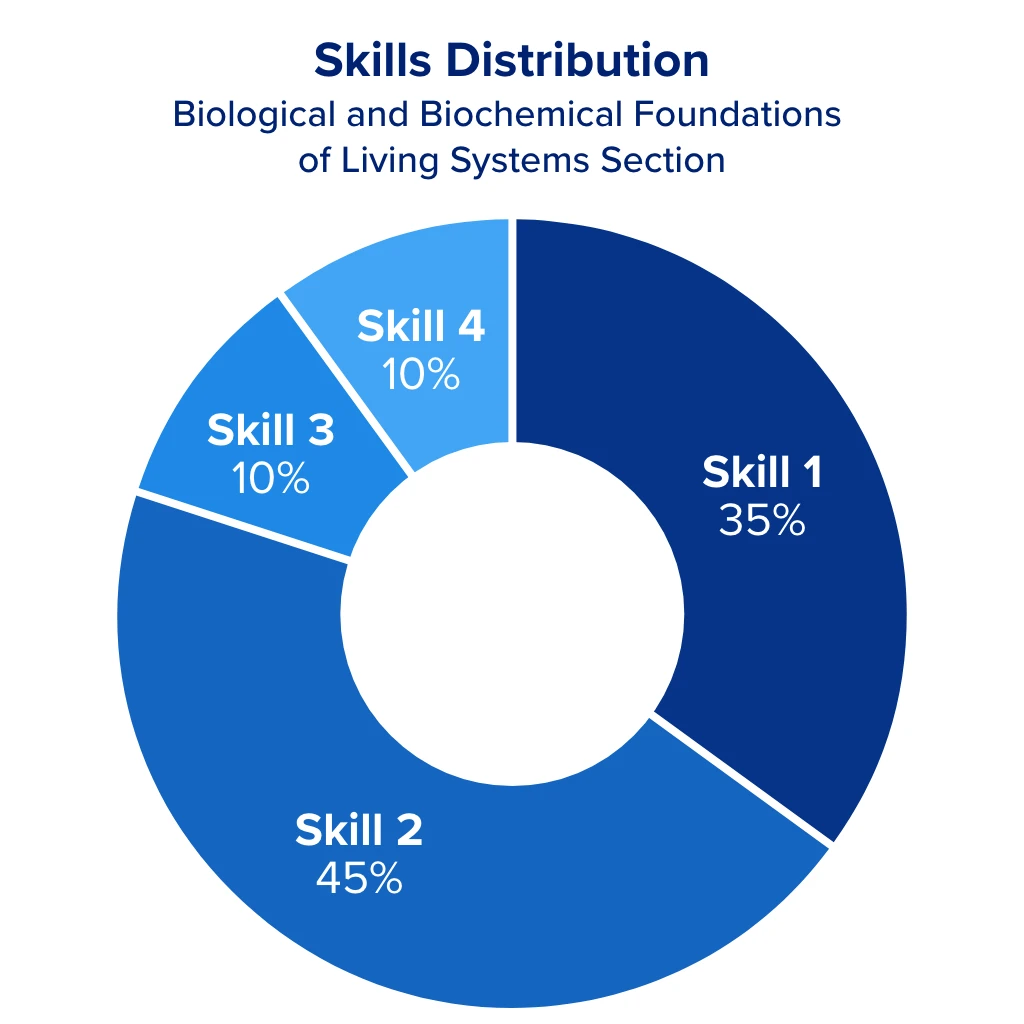
General chemistry makes up 30% of the Chemical and Physical Foundations of Biological Systems section of the MCAT. This equates to roughly 18 questions, or 40 points out of the section’s possible 132. It also makes up 5% of the Biological and Biochemical Foundations of Living Systems section. These combined weights can significantly impact your MCAT score, which is why it’s crucial to understand what you might encounter on the exam.
MCAT General Chemistry Topics
General chemistry on the MCAT is mostly outlined in the AAMC’s Foundational Concepts 4 and 5, with a few individual topics sprinkled in with other subjects. It reflects what you should have learned in a standard two-semester sequence of general chemistry at your university. These principles govern chemical interactions and reactions that impact the molecular dynamics of living systems.
Foundational Concepts 1, 2, and 3
The following are the general chemistry topics examined on the Biological and Biochemical Foundations of Living Systems section of the MCAT (5% of the section).
- Principles of Bioenergetics (includes biochemistry)
- Bioenergetics, thermodynamics
- Free energy, Keq, equilibrium constant, relationship with ΔG°
- Concentration, Le Châtelier’s Principle
- Endothermic and exothermic reactions
- Free energy: G
- Spontaneous reactions and ΔG°
- Phosphoryl group transfers and ATP; ATP hydrolysis ΔG << 0; ATP group transfers
- Biological oxidation-reduction, half-reactions, soluble electron carriers, flavoproteins
- Plasma Membrane
- Solute transport across membranes, osmosis, colligative properties, osmotic pressure
- Electrochemistry
- Concentration cell: direction of electron flow, Nernst equation
- Respiratory System
- Alveolar gas exchange, diffusion, differential partial pressure, Henry’s Law
Foundational Concept 4
The following are the general chemistry topics examined on the Chemical and Physical Foundations of Biological Systems section of the MCAT (30% of the section). The topics below are related to the importance of fluids for the circulation of blood, gas movement, and gas exchange.
- Gas Phase (includes physics)
- Absolute temperature, K, Kelvin scale
- Pressure, simple mercury barometer
- Molar volume at 0°C and 1 atm = 22.4 L/mol
- Ideal Gas Law, Boyle’s Law, Charles’ Law, Avogadro’s Law
- Kinetic Molecular Theory of Gases; heat capacity at constant volume and pressure; Boltzmann’s Constant
- Deviation of real gas behavior from Ideal Gas Law, qualitative and quantitative (Van der Waals’ Equation)
- Partial pressure, mole fraction
- Dalton’s Law relating partial pressure to composition
The following are general chemistry topics related to electrochemistry.
- Electrochemistry
- Electrolytic cell; electrolysis; anode and cathode; electrolyte; Faraday’s Law relating amount of elements deposited (or gas liberated) at an electrode to current; electron flow, oxidation and reduction at the electrodes
- Galvanic or Voltaic cells, half-reactions, reduction potentials, cell potential, direction of electron flow
- Concentration cell
- Batteries, electromotive force, voltage, lead-storage batteries, nickel-cadmium batteries
The following are general chemistry topics related to how light and sound interact with matter.
- Molecular Structure and Absorption Spectra
- Visible region; absorption in visible region gives complementary color (e.g., carotene); effect of structural changes on absorption (e.g., indicators)
The following are general chemistry topics related to atoms, nuclear decay, electronic structure, and atomic chemical behavior.
- Atomic Nucleus (includes physics)
- Atomic number, atomic weight
- Neutrons, protons, isotopes
- Nuclear forces, binding energy
- Radioactive decay; α, β, γ decay; half-life, exponential decay, semi-log plots
- Mass spectrometer
- Mass spectroscopy
- Electronic Structure
- Orbital structure of hydrogen atom, principal quantum number n, number of electrons per orbital
- Conventional notation for electronic structure
- Effective nuclear charge
- The Periodic Table – Classification of Elements Into Groups by Electronic Structure
- Alkali metals
- Alkaline earth metals: their chemical characteristics
- Halogens: their chemical characteristics
- Noble gases: their physical and chemical characteristics
- Transition metals
- Representative elements
- Metals and nonmetals
- Oxygen group
- The Periodic Table – Variations of Chemical Properties with Group and Row
- Valence electrons
- First and second ionization energy, definition, prediction from electronic structure for elements in different groups or rows
- Electron affinity, definition, variation with group and row
- Electronegativity, definition, comparative values for some representative elements and important groups
- Electron shells and the sizes of atoms
- Electron shells and the sizes of ions
- Stoichiometry
- Molecular weight
- Empirical vs molecular formula
- Metric units commonly used in the context of chemistry
- Description of composition by percent mass
- Mole concept, Avogadro’s number NA
- Definition of density
- Oxidation number, common oxidizing and reducing agents, disproportionation reactions
- Description of reactions by chemical equations, conventions for writing chemical equations, balancing equations (including redox equations), limiting reactants, theoretical yields
Foundational Concept 5
The following are general chemistry topics related to the unique nature of water and its solutions.
- Acid-Base Equilibria (includes biochemistry)
- Brønsted-Lowry definition of acid, base
- Ionization of water; Kw and its approximate value (Kw = [H+] [OH–] = 10-14 at 25°C, 1 atm); definition of pH: pH of pure water
- Conjugate acids and bases
- Strong acids and bases
- Weak acids and bases; dissociation of weak acids and bases with or without added salt; hydrolysis of salts of weak acids or bases; calculation of pH of solutions of salts of weak acids or bases
- Equilibrium constants Ka and Kb: pKa, pKb
- Buffers, definition and concepts (common buffer systems), influence on titration curves
- Ions in Solutions (includes biochemistry)
- Anion, cation: common names, formulas, and charges for familiar ions
- Hydration, the hydronium ion
- Solubility
- Units of concentration (e.g., molarity)
- Solubility product constant, the equilibrium expression Ksp
- Common-ion effect and its use in laboratory separations, complex ion formation, complex ions and solubility, solubility and pH
- Titration
- Indicators
- Neutralization
- Interpretation of the titration curves
- Redox titration
The following are general chemistry topics related to the nature of molecules and intermolecular interactions.
- Covalent Bond
- Lewis electron dot formulas, resonance structures, formal charge, Lewis acids and bases
- Partial ionic character, role of electronegativity in determining charge distribution, dipole moment
- σ and π bonds; hybrid orbitals: sp3, sp2, sp, and respective geometries; valence shell electron pair repulsion and the prediction of shapes of molecules; structural formulas for molecules involving H, C, N, O, F, S, P, Si, Cl; delocalized electrons and resonance in ions and molecules
- Multiple bonding, effect on bond length and bond energies, rigidity in molecular structure
- Liquid Phase – Intermolecular Forces
- Hydrogen bonding
- Dipole interactions
- Van der Waals’ Forces (London dispersion forces)
The following are general chemistry topics related to the principles of chemical thermodynamics and kinetics.
- Energy Changes in Chemical Reactions – Thermochemistry, Thermodynamics (includes physics)
- Thermodynamic system – state function
- Zeroth Law – concept of temperature
- First Law – conservation of energy in thermodynamic processes
- Second Law – concept of entropy, as a measure of disorder and relative entropy for gas, liquid, and crystal states
- Measurement of heat changes (calorimetry), heat capacity, specific heat
- Endothermic and exothermic reactions; enthalpy, H, and standard heats of reaction and formation; Hess’ Law of Heat Summation
- Bond dissociation energy as related to heats of formation
- Free energy: G
- Spontaneous reactions and ΔG°
- Heat of fusion, heat of vaporization
- Phase diagram: pressure and temperature
- Rate Processes in Chemical Reactions – Kinetics and Equilibrium
- Reaction rate
- Dependence of reaction rate on concentration of reactants; rate law, rate constant; reaction order
- Rate-determining step
- Dependence of reaction rate on temperature; activation energy, activated complex or transition state, interpretation of energy profiles showing energies of reactants, products, activation energy, and ΔH for the reaction; use of the Arrhenius Equation
- Kinetic control vs thermodynamic control of a reaction
- Catalysts
- Equilibrium in reversible chemical reactions, Law of Mass Action, Equilibrium Constant, application of Le Châtelier’s Principle
- Relationship of the equilibrium constant and ΔG°

What are the MCAT skills tested on General Chemistry questions?
Each MCAT section containing general chemistry will test you on all four of the AAMC’s scientific inquiry and reasoning skills.
Skill 1 requires examinees to:
- Demonstrate their understanding of scientific concepts and principles
- Identify the relationships between closely related concepts
These questions ask you to recognize, identify, recall, or define concepts in the natural, behavioral, and social sciences, and their relation to one another.
Skill 2 requires examinees to:
- Reason about scientific principles, theories, and models
- Analyze and evaluate scientific explanations and predictions
These questions ask you to use scientific knowledge to solve problems in the natural, behavioral, and social sciences.
Skill 3 requires examinees to:
- Demonstrate an understanding of important components of scientific research
- Reason about ethical issues in research
These questions ask you to display your scientific inquiry skills by “doing” science. You must understand scientific methodology and demonstrate your knowledge of the ways natural, behavioral, and social scientists conduct research.
Skill 4 requires examinees to:
- Interpret patterns in data presented in tables, figures, and graphs
- Reason about data and draw conclusions from it
These questions ask you to display your ability to “do” science through data-based and statistical-reasoning skills. You will need to be able to read and interpret results in tables, graphs, and charts, identify patterns in data, and draw conclusions from provided evidence.
With that said, 80% of the questions in these sections are related to skills 1 and 2, meaning most of your general chemistry questions will ask you to:
- Demonstrate your understanding of concepts and principles
- Identify the relationships between closely related concepts
- Reason about principles, theories, and models
- Analyze and evaluate explanations and predictions
The remaining 20% of problems are related to skills 3 and 4, involving research applications and interpreting data, which ask you to:
- Demonstrate an understanding of important components of research
- Reason about ethical issues in research
- Interpret patterns in data presented in tables, figures, and graphs
- Reason about data and draw conclusions from it
MCAT General Chemistry Sample Questions
The following MCAT general chemistry questions and explanations were pulled directly from our MCAT QBank to show how UWorld can help you raise your score. Carefully read each practice question and select your answers to review full rationales.
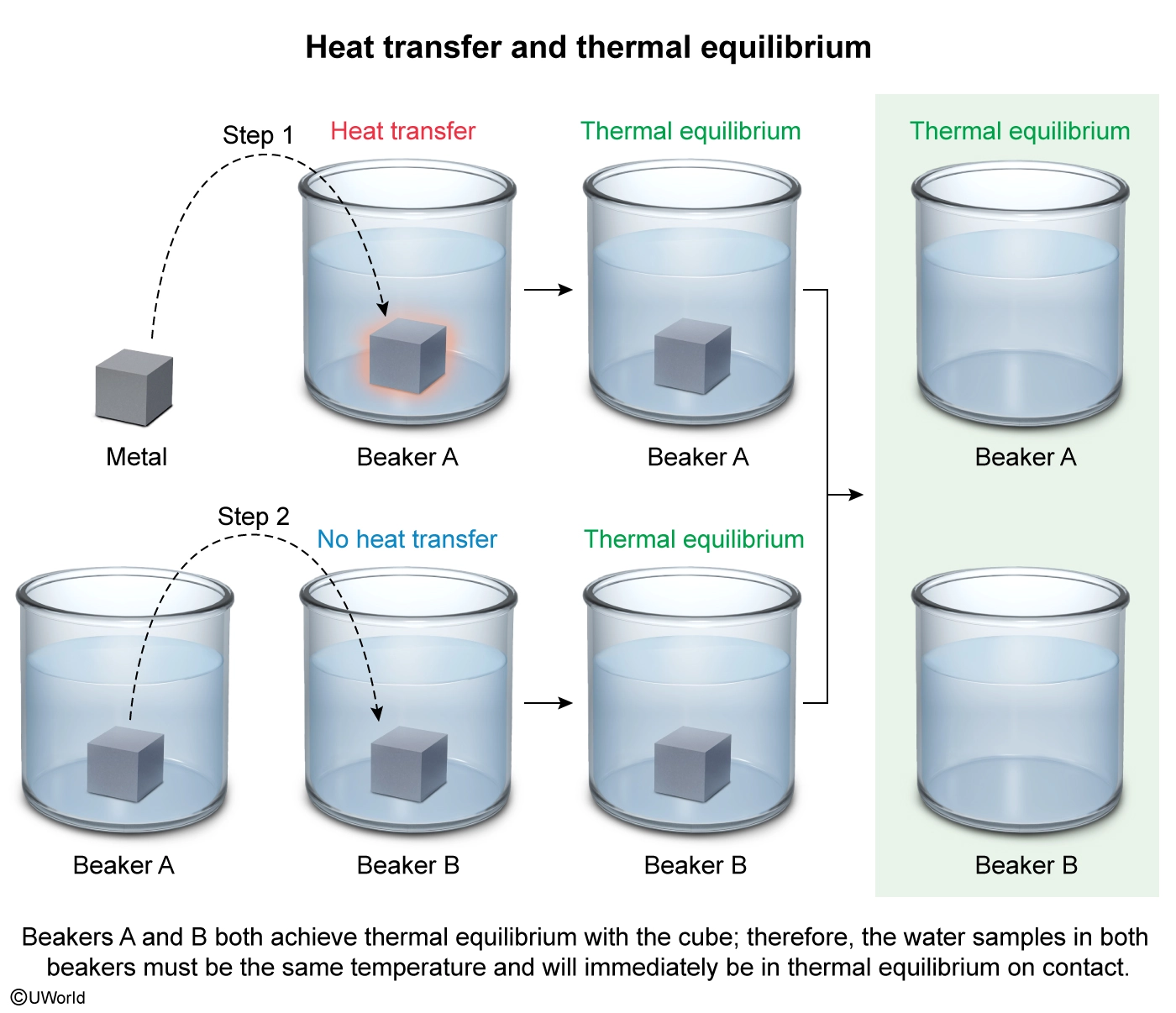
A metal cube with a temperature of 40 °C is placed into 25 mL of water in Beaker A where heat is transferred to the water. After the system reaches thermal equilibrium, the metal cube is then placed into 25 mL of water in Beaker B, but no net heat transfer occurs. The cube is then removed, and the beakers are placed together so that the sides are in thermal contact. Which of the following pairs of objects are in thermal equilibrium at the end of each step of the experiment?
- Beaker A and the metal cube
- Beaker B and the metal cube
- Beaker A and Beaker B
| A. I only | |
| B. II only | |
| C. I and II only | |
| D. I, II, and III |
At temperatures above absolute zero, the atoms and molecules (ie, particles) of a sample of matter are in constant motion (eg, vibration, translation, rotation) and have various amounts of kinetic energy KE (ie, the energy of motion). As such, a sample's temperature is proportional to the average KE of its particles.
When two samples are in thermal contact, atomic and molecular collisions transfer energy (ie, heat) from the sample with higher KE (ie, the warmer sample) to the sample with lower KE (ie, the cooler sample) until the particles in each sample reach thermal equilibrium and have the same average KE (ie, the same temperature). No net transfer of heat occurs between samples at thermal equilibrium.
In this question, the metal cube is initially at a higher temperature than the water in Beaker A until thermal equilibrium is established by the transfer of heat (Number I). When the cube is subsequently placed into the water in Beaker B, no net heat transfers which indicates that the cube and the water are already the same temperature and are immediately in thermal equilibrium on contact (Number II).
According to the zeroth law of thermodynamics, if two systems are in thermal equilibrium, and one of those systems is also in thermal equilibrium with a third system, then all three systems must be in thermal equilibrium with each other (ie, if A ⇄ C and B ⇄ C, then A ⇄ B). Because the cube achieved thermal equilibrium with the water in both beakers, the water in both beakers must be the same temperature and will also be immediately in thermal equilibrium with no net heat transfer with each other on contact (Number III).
Educational objective:
The zeroth law of thermodynamics states that if
two systems are in thermal equilibrium, and one of those systems is also in thermal
equilibrium with a third system, then all three systems must be in thermal equilibrium with
each other.
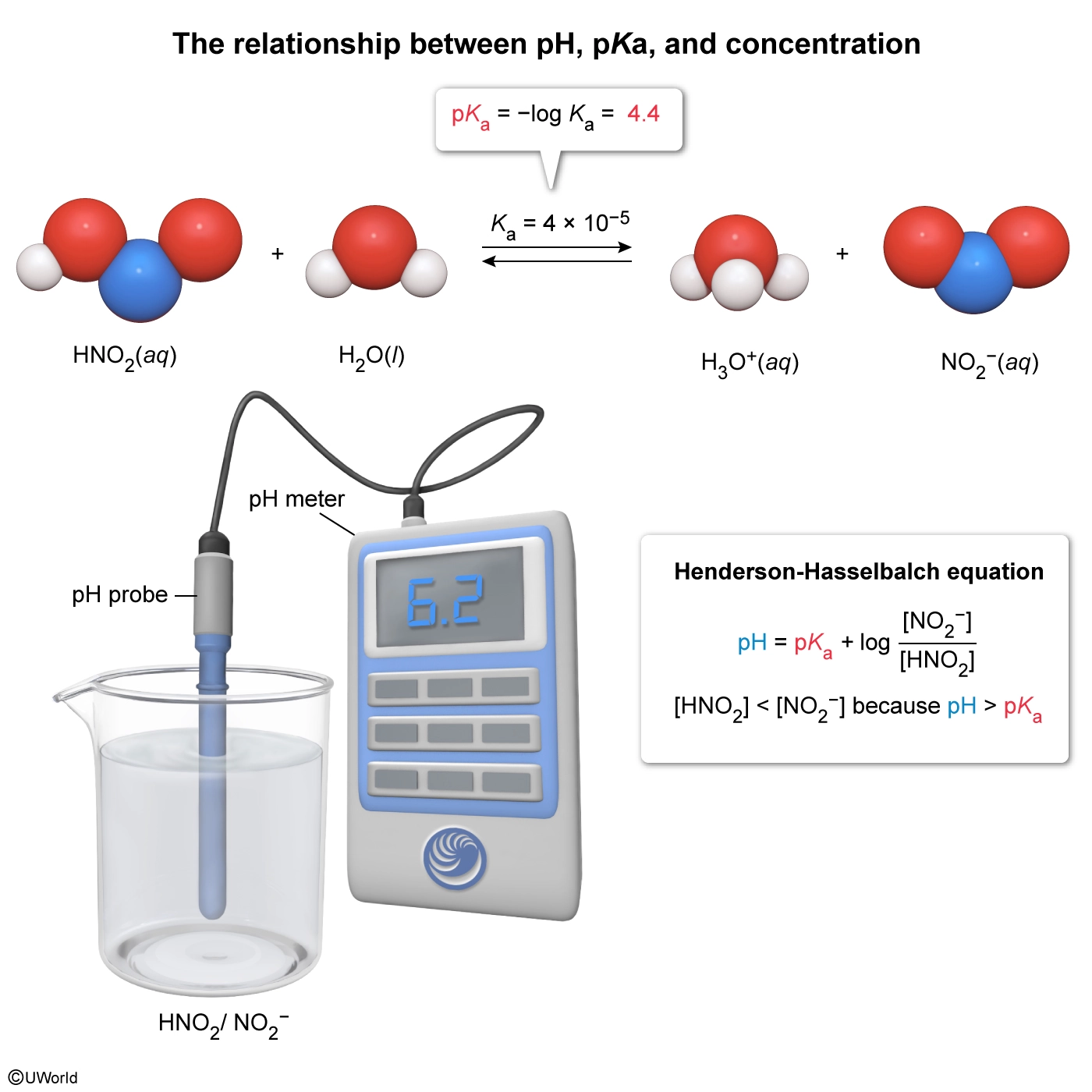
Assume HNO2 dissociates in water with a Ka of 4 × 10−5 at 298 K.
HNO2(aq) ⇄ NO2−(aq) + H3O+(aq)
An aqueous solution containing a mixture of HNO2 and NO2− has a pH of 6.2 at 298 K. Compared to the concentration of NO2−, the concentration of HNO2 must be:
| A. greater, because the pH of the solution is greater than its pKa. | |
| B. less, because the pH of the solution is greater than its pKa. | |
| C. greater, because the pH of the solution is less than its pKa. | |
| D. less, because the pH of the solution is less than its pKa. |
The dissociation of a weak acid (HA) in water can be represented by the general equilibrium reaction:
The extent to which an acid dissociates in water is characterized by the acid dissociation constant Ka, which is also expressed as the pKa:
Without a calculator, the negative logarithm can be approximated using the relationship:
where n is a whole number and m is a number between 1 and 10.
A solution's acidity is measured by its pH, which depends on the molar concentration of H3O+. The relationship between pKa and pH can be quantitatively described by the Henderson-Hasselbalch equation:
If the pH of a solution is less than the pKa (ie, the solution is more acidic), the equilibrium is shifted toward a greater concentration of the protonated acid (ie, [HA] > [A−]). Conversely, if the pH is greater than the pKa (ie, the solution is more basic), the equilibrium is shifted toward a greater concentration of the deprotonated conjugate base (ie, [HA] < [A−]).
The dissociation of HNO2 in water (Ka = 4 × 10−5) proceeds according to the equilibrium reaction:
As such, the pKa is:
Without a calculator, the pKa of HNO2 is approximated as:
The question states that an aqueous solution containing a mixture of HNO2 and NO2− (ie, a buffer) has a pH of 6.2. Therefore, the concentration of HNO2 must be less than NO2− (ie, [HNO2] < [NO2−]) because the pH of the solution is greater than the pKa (ie, 6.2 > 4.4).
(Choices A, C, and D) The pH of the solution is greater than the pKa of HNO2; consequently, less HNO2 is present in the solution compared to NO2−.
Educational objective:
The pKa is a measure of the acidity of a
particular proton in a molecule. In a solution containing a weak acid (HA) and its conjugate base
(A−), when the pH is greater than the pKa, the amount HA is less than the amount
of A− and vice versa.
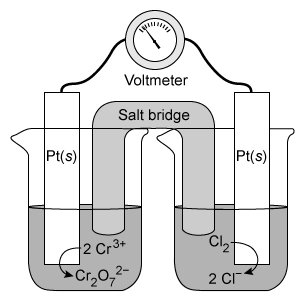
Which species is oxidized as the galvanic cell operates?
| A. Cr3+ | |
| B. Cl2 | |
| C. Cl− | |
| D. Cr2O72− |
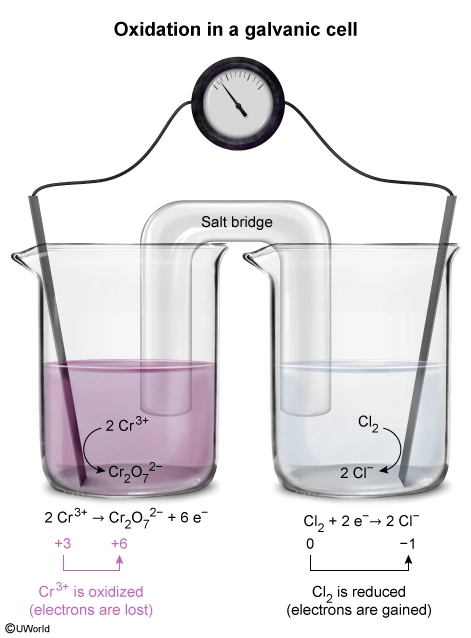
During an oxidation-reduction (redox) reaction, electrons are transferred from one atom to another. The atom that loses electrons is oxidized, and the atom that gains electrons is reduced. The oxidation state of the oxidized atom increases by the number of electrons lost, and the oxidation state of the reduced atom decreases by the number of electrons gained. Oxidation states can be determined using the compound formula and oxidation state rules. The sum of all the oxidation states of each atom in the compound must be equal to the net charge of the compound.
In the galvanic cell shown in the question, the Cr atom has an initial oxidation state of +3 (ie, Cr3+). Applying the oxidation state rules to the Cr2O72− anion shows that the final oxidation state of the Cr atom is +6. Although Cr is a transition state metal with a variable oxidation state, the oxidation state of O (−2) is consistent and can be used to determine the oxidation state of the Cr atoms. Because Cr2O72− has a net charge of −2, the oxidation states of Cr and O must sum to −2.
Cr2O72− = −2
2(Cr) + 7(−2) = −2
2(Cr) − 14 = −2
Cr = +6
Therefore, Cr3+ is oxidized (ie, 3 electrons lost to form Cr6+ in Cr2O72−) as the galvanic cell operates.
(Choices B and C) The oxidation state of the Cl atom increases (ie, becomes more negative) from 0 (Cl2) to −1 (Cl−). As such, Cl2 is reduced, not oxidized.
(Choice D) Cr2O72− results from the oxidation of Cr3+ and is not itself oxidized.
Educational objective:
The oxidation state of each atom in a compound
or ion formula can be determined using oxidation state rules. The sum of the oxidation
states of each atom must equal the net charge of the compound or ion. An increased oxidation
state indicates a loss of electrons (oxidation) whereas a decreased oxidation state results
from gaining electrons (reduction).
MCAT General Chemistry Tips and Strategies
Because general chemistry on the MCAT is very application-based, we recommend practicing as many quality questions as you can while reviewing associated answer explanations. This will reinforce the chemistry equations and topics you need to know for the exam. The UWorld MCAT QBank is an excellent resource to receive full content coverage, exam-like questions, detailed explanations, and innovative learning tools.