MCAT® Organic Chemistry
Topics, Practice Questions, and Study Strategies

MCAT Organic Chemistry Topics
The MCAT covers organic chemistry through the AAMC's Foundational Concepts 1, 3, 4, and 5. During medical school, students apply the fundamental principles of organic chemistry to comprehend crucial aspects of medicine, such as biochemistry, drug development, and drug design. Only some things you may have covered in two semesters of Organic Chemistry will appear on the MCAT. Below are the organic chemistry topics you need to know:
Foundational Concept 1
The following are organic chemistry topics relevant to the structure and function of proteins and amino acids.
- Amino Acids
- Description
- Absolute configuration at the α position
- Amino acids as dipolar ions
- Classifications
- Acidic or basic
- Hydrophobic or hydrophilic
- Reactions
- Sulfur linkage for cysteine and cystine
- Peptide linkage: polypeptides and proteins
- Hydrolysis
- Description
- Protein Structure
- Structure
- 1° structure of proteins
- 2° structure of proteins
- 3° structure of proteins; role of proline, cystine, hydrophobic bonding
- Conformational stability
- Denaturing and folding
- Hydrophobic interactions
- Separation techniques
- Isoelectric point
- Electrophoresis
- Structure
The following are organic chemistry topics relevant to the study of bioenergetics and fuel molecule metabolisms.
- Carbohydrates
- Description
- Nomenclature and classification, common names
- Absolute configuration
- Cyclic structure and conformations of hexoses
- Epimers and anomers
- Hydrolysis of the glycosidic linkage
- Monosaccharides
- Disaccharides
- Polysaccharides
- Description
Foundational Concept 3
The following are organic chemistry topics relevant to the nervous and endocrine systems’ structure, function, and coordination.
- Lipids
- Description; structure
- Steroids
- Terpenes and terpenoids
- Description; structure
Foundational Concept 4
The following are organic chemistry topics relevant to the interaction of light and sound with matter.
- Molecular Structure and Absorption Spectra
- Infrared region
- Intramolecular vibrations and rotations
- Recognizing common characteristic group absorptions, fingerprint region
- Visible region (GC)
- Absorption in visible region gives complementary color (e.g., carotene)
- Effect of structural changes on absorption (e.g., indicators)
- Ultraviolet region
- π-Electron and nonbonding electron transitions
- Conjugated systems
- NMR spectroscopy
- Protons in a magnetic field; equivalent protons
- Spin-spin splitting
- Infrared region
Foundational Concept 5
The following are organic chemistry topics relevant to the separation and purification of mixtures.
- Separations and Purifications
- Extraction: distribution of solute between two immiscible solvents
- Distillation
- Chromatography: basic principles involved in separation process
- Column chromatography
- Gas-liquid chromatography
- High-pressure liquid chromatography
- Paper chromatography
- Electrophoresis
- Quantitative analysis
- Chromatography
- Size-exclusion
- Ion-exchange
- Affinity
- Column chromatography
- Racemic mixtures, separation of enantiomers
The following are organic chemistry topics related to biologically relevant molecules’ structure, function, and reactivity.
- Amino Acids, Peptides, Proteins
- Amino acids: description
- Absolute configuration at the α position
- Dipolar ions
- Classification
- Acidic or basic
- Hydrophilic or hydrophobic
- Synthesis of α-amino acids
- Strecker Synthesis
- Gabriel Synthesis
- Peptides and proteins: reactions
- Sulfur linkage for cysteine and cystine
- Peptide linkage: polypeptides and proteins
- General principles
- Primary structure of proteins
- Secondary structure of proteins
- Tertiary structure of proteins
- Isoelectric point
- Amino acids: description
- Lipids
- Description, types
- Storage
- Triacylglycerols
- Free fatty acids: saponification
- Structural
- Phospholipids and phosphatides
- Waxes
- Signals, cofactors
- Fat-soluble vitamins
- Steroids
- Storage
- Description, types
- Carbohydrates
- Description
- Nomenclature and classification, common names
- Absolute configuration
- Cyclic structure and conformations of hexoses
- Epimers and anomers
- Hydrolysis of the glycosidic linkage
- Keto-enol tautomerism of monosaccharides
- Description
- Aldehydes and Ketones
- Description
- Nomenclature
- Physical properties
- Important reactions
- Nucleophilic addition reactions at C=O bond
- Acetal, hemiacetal
- Imine, enamine
- Hydride reagents
- Cyanohydrin
- Oxidation of aldehydes
- Reactions at adjacent positions: enolate chemistry
- Keto-enol tautomerism (α-racemization)
- Aldol condensation, retro-aldol
- Kinetic vs. thermodynamic enolate
- General principles
- Effect of substituents on reactivity of C=O; steric hindrance
- Acidity of α-H; carbanions
- Nucleophilic addition reactions at C=O bond
- Description
- Alcohols
- Description
- Nomenclature
- Physical properties (acidity, hydrogen bonding)
- Important reactions
- Oxidation
- Substitution reactions: SN1 or SN2
- Protection of alcohols
- Preparation of mesylates and tosylates
- Description
- Carboxylic Acids
- Description
- Nomenclature
- Physical properties
- Important reactions
- Carboxyl group reactions
- Amides (and lactam), esters (and lactone), anhydride formation
- Reduction
- Decarboxylation
- Reactions at 2-position, substitution
- Carboxyl group reactions
- Description
- Acid Derivatives (Anhydrides, Amides, Esters)
- Description
- Nomenclature
- Physical properties
- Important reactions
- Nucleophilic substitution
- Transesterification
- Hydrolysis of amides
- General principles
- Relative reactivity of acid derivatives
- Steric effects
- Electronic effects
- Strain (e.g., β-lactams)
- Description
- Phenols
- Oxidation and reduction (e.g., hydroquinones, ubiquinones): biological 2e– redox centers
- Polycyclic and Heterocyclic Aromatic Compounds
- Biological aromatic heterocycles
What are the MCAT skills tested on Organic Chemistry questions?
Each MCAT section containing organic chemistry will test you on all four of the AAMC’s scientific inquiry and reasoning skills.
Skill 1 requires examinees to:
- Demonstrate their understanding of scientific concepts and principles
- Identify the relationships between closely related concepts
These questions ask you to recognize, identify, recall, or define concepts in the natural, behavioral, and social sciences, and their relation to one another.
Skill 2 requires examinees to:
- Reason about scientific principles, theories, and models
- Analyze and evaluate scientific explanations and predictions
These questions ask you to use scientific knowledge to solve problems in the natural, behavioral, and social sciences.
Skill 3 requires examinees to:
- Demonstrate an understanding of important components of scientific research
- Reason about ethical issues in research
These questions ask you to display your scientific inquiry skills by “doing” science. You must understand scientific methodology and demonstrate your knowledge of the ways natural, behavioral, and social scientists conduct research.
Skill 4 requires examinees to:
- Interpret patterns in data presented in tables, figures, and graphs
- Reason about data and draw conclusions from it
These questions ask you to display your ability to “do” science through data-based and statistical-reasoning skills. You will need to be able to read and interpret results in tables, graphs, and charts, identify patterns in data, and draw conclusions from provided evidence.
Most of the organic chemistry questions in both sections are related to skills 1 and 2. Most of your organic chemistry questions will ask you to:
- Demonstrate your understanding of concepts and principles
- Identify the relationships between closely related concepts
- Reason about principles, theories, and models
- Analyze and evaluate explanations and predictions
The remaining 20% are related to skills 3 and 4, involving research applications and interpreting data, which ask you to:
- Demonstrate an understanding of essential components of research
- Reason about ethical issues in research
- Interpret patterns in data presented in tables, figures, and graphs
- Reason about data and draw conclusions from it
How is Organic Chemistry tested on the MCAT?
Organic chemistry MCAT questions may be stand-alone discrete or passage-based questions. Discrete questions test your content knowledge on specific topics you should know off the top of your head. Passage-based questions test your analytical and critical thinking skills and your content knowledge.
The passage can be a synthesis experiment or a type of purification experiment. In these passages, you may have to deduce the mechanism under which specific reagents react. You could also be tested on functional group identity. Given the conditions described in the question stem, you may have to identify a particular spectroscopy test. The question stem may require you to identify and differentiate between the best type of chromatography to separate and purify a compound.
MCAT Organic Chemistry Sample Questions
The following MCAT organic chemistry questions and explanations were pulled directly from our MCAT QBank to show how UWorld can help you raise your score. Carefully read each practice question and select your answers to review full rationales.
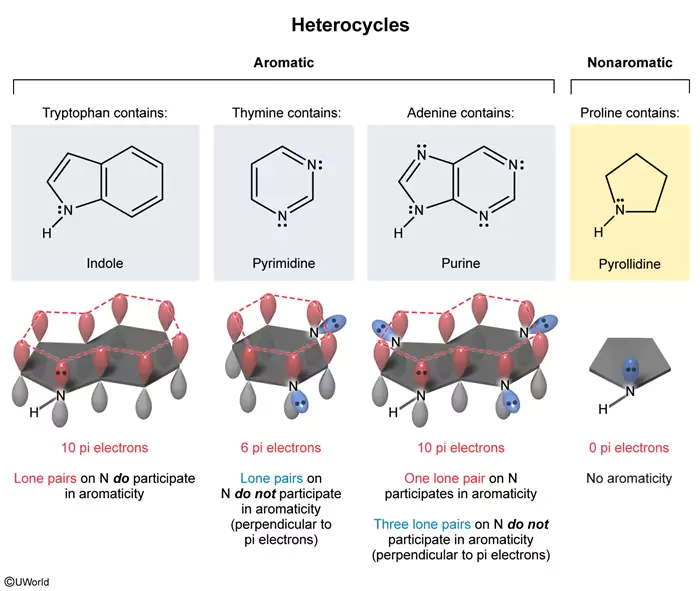
All of the following can be described as an aromatic heterocycle EXCEPT:
| A. tryptophan. | |
| B. proline. | |
| C. thymine. | |
| D. adenine. |
Aromatic heterocycles are commonly found in biological systems. A heterocycle is any cyclic compound containing at least one heteroatom (ie, an atom other than carbon or hydrogen). To be classified as aromatic, a compound must have:
- Conjugated pi bonds in a cyclic structure
- Unhybridized p orbitals present in each atom in the ring
- Planar geometry, forming a continuous ring of parallel, overlapping unhybridized p orbitals
- 4n + 2 pi electrons (Hückel rule), where n is a non-negative integer
Tryptophan, thymine, and adenine are all considered aromatic heterocycles because they have nitrogen-containing rings (indole, pyrimidine, and purine, respectively) with conjugated pi bonds and overlapping unhybridized p orbitals (Choices A, C, and D). Although thymine may not appear to have conjugated pi bonds, a resonance structure can be drawn to show a conjugated system similar to pyrimidine. Basic nitrogen atoms, such as in pyrimidine and purine, have a double bond placing the lone pair electrons in an sp2 orbital perpendicular to the conjugated p orbitals containing the pi electrons. Electrons in orbitals perpendicular to the conjugated plane cannot undergo side-to-side overlap with the p orbitals and therefore do not participate in aromaticity. Nonbasic nitrogen atoms, such as in purine and indole, have three bonds, putting the lone pair of electrons into a p orbital parallel to other pi electron-containing p orbitals. Parallel orbitals permit side-to-side overlap; therefore, these electrons do participate in aromaticity. As a result, thymine has 6 pi electrons, and tryptophan and adenine each have 10 pi electrons; all satisfy Hückel rule.
(Choice B) Proline can be classified as a heterocycle, but it is not considered aromatic because it is not conjugated and does not contain any pi electrons.
Educational objective:
To be considered aromatic, a compound must be planar and cyclic with conjugated pi bonds. Each atom must have parallel, overlapping unhybridized p orbitals. Finally, an aromatic compound will satisfy Hückel rule and have 4n + 2 pi electrons.
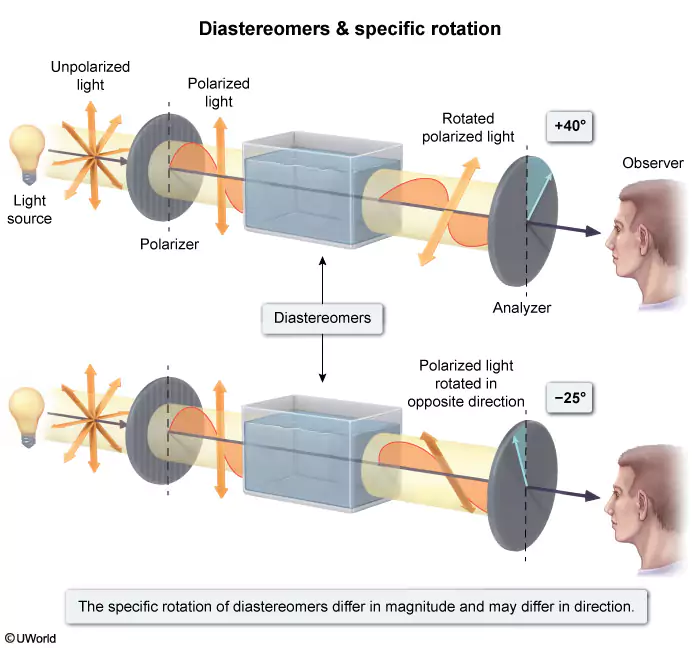
Which term can be used to classify the relationship between two isomers that have the same connectivity but specific rotations of +40° and −25°, respectively?
| A. Enantiomers | |
| B. Racemic mixture | |
| C. Diastereomers | |
| D. Conformational isomers |
Specific rotation, the degree to which chiral molecules rotate plane-polarized light, is unique to each chiral molecule. The value of specific rotation consists of direction (+ or −) and magnitude (number of degrees). Clockwise rotations are designated as (+), whereas counterclockwise rotations are (−).
Isomers are molecules with the same molecular formula but have either different connectivity (constitutional isomers) or spatial orientation (stereoisomers). Diastereomers differ at one or more stereocenters but have the orientation of at least one stereocenter in common. The specific rotations of diastereomers differ in magnitude and may differ in direction. The same is true for constitutional isomers.
Because the question states that the two molecules have the same connectivity, they cannot be constitutional isomers. Because they have different specific rotations (direction and magnitude), it can be concluded that the two molecules are diastereomers.
(Choice A) Enantiomers have specific rotations of equal magnitude but opposite directions. If the molecules were enantiomers, they would either have specific rotations of +40° and −40° or +25° and −25°.
(Choice B) A racemic mixture is composed of 50% of one enantiomer and 50% of the other. Racemic mixtures have a specific rotation of 0° because the specific rotations of the two isomers cancel each other. Each isomer in the question has a nonzero specific rotation and therefore cannot be racemic.
(Choice D) Conformational isomers are versions of the same molecule that form when an atom rotates about its bond. Because conformational isomers are forms of the same compound, they will have the same specific rotation rather than different specific rotations.
Educational objective:
Specific rotation is the degree to which a chiral molecule rotates plane-polarized light. The value of specific rotation contains direction (+ is clockwise, − is counterclockwise) and magnitude. Diastereomers differ in the magnitude of their specific rotations (and may differ in direction), whereas enantiomers have specific rotations of the same magnitude but opposite direction.
| A. HCD | |
| B. HME | |
| C. WME | |
| D. WMD |
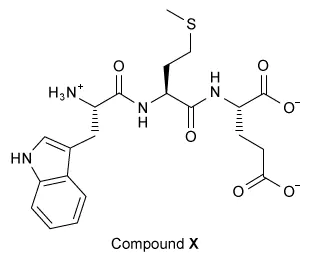
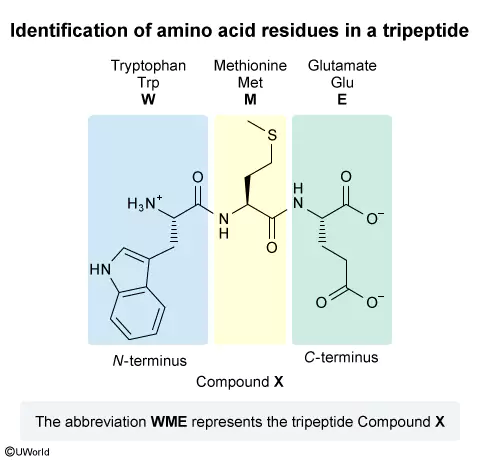
The structure of an amino acid consists of a carboxyl group, an amino group, and a side chain, all bonded to the same carbon atom (the α-carbon). The main difference in the structure of the 20 common amino acids is the side chain, which has different functional groups. Amino acids are classified by the nature of the side chains (eg, hydrophilic, hydrophobic, basic, acidic) and can be named using shorthand one- and three-letter abbreviations. Polypeptide names list the amino acid residues from the end containing the free α-amino group (the N-terminus) to the end containing the free carboxyl group (the C-terminus).
In this question, Compound X is a tripeptide, which has three amino acid residues. The N-terminus is on the left side of the structure, and the C-terminus is on the right side. From the N-terminus terminus, the first residue contains an indole group and is tryptophan (Trp, W). The second residue has a side chain containing a thioether group and is methionine (Met, M). The residue at the C-terminus has a side chain containing a carboxylate group and a total of three carbon atoms (including the carboxylate group) and is glutamate (Glu, E).
Therefore, the abbreviation that corresponds to Compound X is WME.
(Choice A) HCD corresponds to a tripeptide containing the residues histidine (His, H), whose side chain contains an imidazole; cysteine (Cys, C), whose side chain contains a thiol; and aspartate (Asp, D), which contains a carboxylate side chain. These residues are similar (but not the same) to those in Compound X.
(Choice B) The first residue in HME is incorrect. H corresponds to histidine, the amino acid with a heterocyclic imidazole group; however, the first residue in Compound X is tryptophan (W), which contains a heterocyclic indole group.
(Choice D) The final residue in WMD is incorrect. D corresponds to aspartate, whose side chain contains one less CH2 group than glutamate (E), the correct final residue in Compound X.
Educational objective:
Amino acids contain a carboxyl group, an amino group, and a side chain, all bonded to the same carbon atom. The structure of amino acids differs mainly with the functional groups contained in the side chain. In addition to their chemical names, amino acids have one- and three-letter abbreviations.
Organic Chemistry Tips and Strategies
Because MCAT questions are application-based, we recommend practicing with representative organic chemistry MCAT questions and reviewing their associated answer explanations. This will reinforce the topics you need to know for the exam. The UWorld MCAT QBank is an excellent resource for receiving full content coverage, exam-like questions, detailed explanations, and innovative learning tools. The explanations include rationales for correct and incorrect answers and highlight where the answer is in each passage. Each explanation also includes a high-yield visual that explains the content for visual learners.
Learn by Doing
Students often struggle with organic chemistry passages. To improve, it is imperative that you put yourself in the testing environment and practice organic chemistry passages. Active learning is a tool that can help you improve your ability to complete MCAT passages. You can harness the power of active learning by completing practice questions and reviewing the explanations. This active learning approach is more effective than reading books cover to cover because you are actively testing your skills and improving. Our MCAT sample questions challenge you to apply your content knowledge and test your critical thinking skills in an exam-like setting.
Use the Answer Choices to Figure Out Mechanisms
Students often need help with questions about mechanisms. The MCAT will require you to deduce which functional groups have been removed or added based on inferences from the figure. This is where you can work backward. Plug the answer choice into the figure to see if the mechanism could be possible with the answer choice.
Read the Question Stem Carefully
This last tip is the most important. You must fully understand what the question stem is asking you. If you are completing a passage, the question stem will contain important keywords that will lead you to the answer within the passage. In a discrete question, the question stem keywords will prompt you to remember specific ideas and concepts based on your content knowledge that will lead you to the correct answer. The MCAT includes hints within each question stem.
Frequently Answered Questions
How many organic chemistry questions are on the MCAT?
What lab techniques are on the MCAT?
The Organic Chemistry lab techniques tested on the MCAT include:
- Spectroscopy: tests that seek to identify the functional groups of the molecule.
- Infrared spectroscopy
- UV spectroscopy
- Proton NMR.
- Purification: tests that seek to separate different chemical compounds from one another.
- Extraction
- Filtration
- Chromatography and all of its subtypes
- Distillation
- Simple distillation
- Vacuum distillation
- Fractional distillation
- Recrystallization
- Electrophoresis.
- Chromatography: purification tests that separate compounds based on the mobile and the stationary phase.
- Thin layer chromatography (TLC)
- Normal phase high-performance liquid chromatography (HPLC)
- Reverse phase high-performance liquid chromatography (HPLC)
- Column chromatography
- Ion exchange chromatography
- Cation exchange chromatography
- Anion exchange chromatography
- Size exclusion chromatography
- Affinity chromatography
- Gas chromatography
Are mechanisms tested on the MCAT?
Yes, there are quite a few mechanisms that you do need to be familiar with to do well on the organic chemistry section of the MCAT. Some notable mechanisms you need to know are SN1 reactions, SN2 reactions, aldol condensation and formation of enolates, transesterification, nucleophilic, acyl substitution, decarboxylation and carboxylation, hydrolysis reactions, and saponification.
Knowing the mechanism of Strecker synthesis and Gabriel synthesis can be beneficial. However, given how infrequent these two topics appear, it is only worth studying these mechanisms in detail once you’ve scored highly on previous practice tests.
Do you need to memorize organic chemistry reactions for the MCAT?
You don’t need to memorize specific organic chemistry reactions on the MCAT. However, you need to be familiar with the general processes of how these reactions occur. You need to understand how mechanisms function in different scenarios. For example, an SN1 reaction in one MCAT passage may not be the same in another passage because you have different functional groups substituted in the molecule in which the SN1 reaction is reacting. You need to be familiar with the mechanism of the SN1 reaction and how the mechanism will apply to both questions. You don’t need to memorize specific reactions, but you need to learn the general mechanisms to answer all the questions about reactions successfully.
An excellent way to familiarize yourself with reactions is to get a whiteboard, iPad, or sheet of paper and practice rewriting and drawing the mechanisms. Seek to understand why certain electrons attack other groups. The principle of electronegativity is an organic chemistry topic that can help you better understand why certain groups attack others. More electronegative elements tend to want to gain electrons compared to other elements. This causes dipole shifts that are helpful when determining a reaction’s mechanism.